Latest News
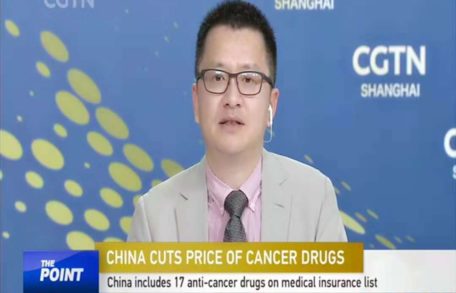
Y. Mark Xu talks with CGTN (China Global TV Network)
Image 10/26/2018 news adminMark Xu, Founder, Chairman, Chief Executive Officer and President of Xynomic talks with CGTN “The Point with Liu Xin” and provides insight regarding the current trend of the price of cancer drugs in China. “The
Details
Xynomic Pharma Receives Fast-Track Designation from the US FDA for Abexinostat as 4L Therapy Treating Follicular Lymphoma
09/26/2019 news adminSeptember 23, 2019 06:00 ET | Source: Xynomic Pharmaceuticals Holdings, Inc. SHANGHAI, Sept. 23, 2019 (GLOBE NEWSWIRE) — Xynomic Pharmaceuticals Holdings, Inc. (“Xynomic”, stock ticker: XYNO), a clinical stage US-China oncology drug development company, today
Details
Xynomic Pharma Doses First Chinese Patient in Pivotal Phase 3 Kidney Cancer Trial and Hires Medical Monitor
09/26/2019 news adminSeptember 13, 2019 15:58 ET | Source: Xynomic Pharmaceuticals Holdings, Inc. SHANGHAI, Sept. 13, 2019 (GLOBE NEWSWIRE) — Xynomic Pharmaceuticals Holdings, Inc. (“Xynomic”, stock ticker: XYNO), a clinical stage US-China oncology drug development company, today
Details
Xynomic Pharma Reports Encouraging Interim Data from Phase 1b Study of Abexinostat Combined with Keytruda® in Multiple Solid Tumors
09/26/2019 news adminAugust 30, 2019 08:41 ET | Source: Xynomic Pharmaceuticals Holdings, Inc. RALEIGH, N.C. and SHANGHAI, Aug. 30, 2019 (GLOBE NEWSWIRE) — Xynomic Pharmaceuticals Holdings, Inc. (“Xynomic”, stock ticker: XYNO), a clinical stage US-China oncology drug
Details
Xynomic Pharma Reports Encouraging Interim Data from Phase 1b Study of Abexinostat Combined with Keytruda® in Multiple Solid Tumors
09/01/2019 news adminAugust 30, 2019 08:41 ET | Source: Xynomic Pharmaceuticals Holdings, Inc. RALEIGH, N.C. and SHANGHAI, Aug. 30, 2019 (GLOBE NEWSWIRE) — Xynomic Pharmaceuticals Holdings, Inc. (“Xynomic”, stock ticker: XYNO), a clinical stage US-China oncology drug
Details
Xynomic Research Institute Has Successfully Designed a Series of Potent RET Kinase Inhibitors
08/20/2019 news adminAugust 16, 2019 16:30 ET | Source: Xynomic Pharmaceuticals Holdings, Inc. RALEIGH, N.C. and SHANGHAI, Aug. 16, 2019 (GLOBE NEWSWIRE) — Xynomic Pharmaceuticals Holdings, Inc. (“Xynomic”, stock ticker: XYNO), a clinical stage US-China oncology drug
Details
Xynomic Pharma Receives Important Accreditation from Independent Ethics Committees of Leading Chinese Cancer Hospitals
08/14/2019 news adminAugust 07, 2019 16:00 ET | Source: Xynomic Pharmaceuticals Holdings, Inc. RALEIGH, N.C. and SHANGHAI, Aug. 07, 2019 (GLOBE NEWSWIRE) — Xynomic Pharmaceuticals Holdings, Inc. (“Xynomic”, stock ticker: XYNO), a clinical stage US-China oncology drug
Details
Xynomic Filed Fast-Track Designation Application for Follicular Lymphoma Treatment with the U.S. FDA
08/14/2019 Uncategorized adminAugust 01, 2019 07:00 ET | Source: Xynomic Pharmaceuticals Holdings, Inc. RALEIGH, N.C. and SHANGHAI, Aug. 01, 2019 (GLOBE NEWSWIRE) — Xynomic Pharmaceuticals Holdings, Inc. (“Xynomic”, stock ticker: XYNO), a clinical stage U.S.-China oncology drug
Details
Xynomic Received China Approval to Start 2 Pivotal Lymphoma Clinical Trials
07/31/2019 Uncategorized adminJuly 30, 2019 07:00 ET | Source: Xynomic Pharmaceuticals Holdings, Inc. RALEIGH, N.C. and SHANGHAI, July 30, 2019 (GLOBE NEWSWIRE) — Xynomic Pharmaceuticals Holdings, Inc. (“Xynomic”, stock ticker: XYNO), a clinical stage US-China oncology drug
Details
Xynomic Pharma Will Present at 3rd World-China Immunotherapy & Gene Therapy Congress
07/26/2019 news adminJuly 26, 2019 12:29 ET | Source: Xynomic Pharmaceuticals Holdings, Inc. RALEIGH, N.C. and SHANGHAI, July 26, 2019 (GLOBE NEWSWIRE) — Xynomic Pharmaceuticals Holdings, Inc. (“Xynomic”, stock ticker: XYNO), a clinical stage U.S.-China oncology drug
Details
Xynomic Completes Pre-IND Meeting with US FDA for XP-102, a Novel Pan-RAF Inhibitor against Colorectal Cancer and Lung Cancer
06/14/2019 news adminRALEIGH, N.C. and SHANGHAI, June 12, 2019 (GLOBE NEWSWIRE) — Xynomic Pharmaceuticals Holdings, Inc. (“Xynomic”, Nasdaq: XYN), a clinical stage US-China oncology drug development company, announced that it recently held a pre-IND meeting with the
Details